Technology
JSK Therapeutics™ is developing new agents for the treatment of several cancers.
Nitric Oxide Biology
Nitric oxide (NO) is a reactive radical with different biological functions including vasodilation, neurotransmission and macrophage-mediated immune response. It is generated from molecular oxygen and L-arginine in a reaction catalyzed by the NO synthases. High amounts of NO (immune cell-generated, or pharmacologically-delivered) inhibit tumor growth and metastasis. However, because of its pleotropic effects, NO cannot be directly administered for the treatment of malignant diseases. To accomplish that purpose, it is therefore necessary to deliver the NO payload in a targeted fashion.
Nitric Oxide Donor Drugs
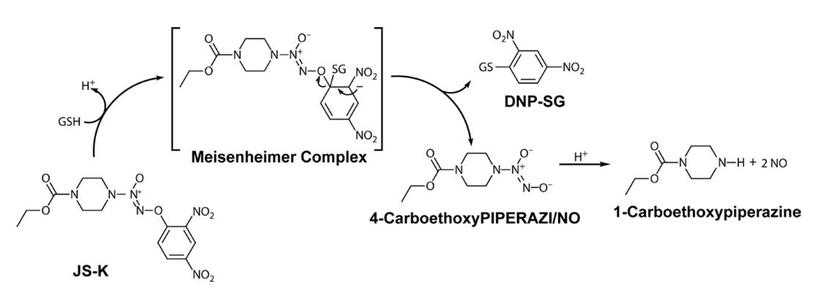
Dr. Larry Keefer’s team at the National Cancer Institute created the class of arylated diazeniumdiolates to target NO release to cancer cells. Arylated diazeniumdiolates do not release large amounts of NO spontaneously. However, upon nucleophilic attack, the diazeniumdiolate is released from the parent molecule and generates NO. Glutathione (GSH) is the primary nucleophile to react with arylated diazeniumdiolates. While the reaction can occur spontaneously, it is catalyzed by the Glutathione S-Transferases (GST). GSTs are upregulated in many cancer cells, including the majority of acute myeloid leukemia (AML) cells. By exploiting an enzyme family that is upregulated in malignant cells, arylated diazeniumdiolates provide an element of selective delivery of NO to the tumor target.
JS-K
The Keefer team synthesized a library of rationally designed arylated diazeniumdiolates that were screened in the Shami lab at the University of Utah for anti-AML activity using HL-60 cells. From that screen, O2-(2,4-dinitrophenyl) 1-[(4 ethoxycarbonyl)piperazin-1-yl] diazen-1-ium-1,2-diolate, or JS-K was identified as the most active agent. Through work in the Shami lab and at different institutions, JS-K has shown single agent activity in animal models of acute myeloid leukemia (AML), multiple myeloma (MM), non-small cell lung cancer, hepatocellular carcinoma, prostate cancer, glioma and Ewing’s sarcoma. JS-K was also shown to inhibit metastasis development in an orthotopic renal cell carcinoma model. JS-K synergizes with cytarabine against AML and with bortezomib against MM cells. Besides its direct cytotoxic effects, JS-K inhibits tumor angiogenesis. It also inhibits the interaction between MM cells and the bone marrow microenvironment. Mechanistically, JS-K impairs the redox status of malignant cells by depleting intracellular GSH. JS-K was also shown to upregulate the expression of CD155 on the surface of MM cells, which could sensitize them to the cytotoxic effects of immune effector cells (natural killer cells). The latter observation raises the possibility of synergy between JS-K and new cancer immunotherapy strategies. Using nanoparticles, the Shami lab has developed a clinically relevant formulation for JS-K. JS-K was well tolerated without induction of hypotension in a non-GLP dog toxicology study.
JS-K is a first-in- class anti-cancer drug that is unique in many respects. It is directly cytotoxic by different mechanisms including impairment of tumor metabolism. JS-K inhibits tumor angiogenesis. JS-K prevents tumor cells from interacting with their microenvironment. Finally, JS-K could sensitize malignant cells to immune effector cells. Besides its effects by itself, these multiple mechanisms of action support the development of potent new drug combinations with approved cytotoxic drugs and immune modulating agents. JS-K is a first-in- class anti-cancer agent with the potential to constitute a paradigm shift in cancer therapy.
Acute Myeloid Leukemia
Acute Myeloid Leukemia (AML) is the most common acute leukemia in adults. SEER estimates indicate that 19,900 cases of AML were diagnosed in the United States in 2020 with 11,180 AML-related deaths. In spite of the recent development of several new agents for AML depending on prognostic factors, cures are achievable in a minority of patients, but at the cost of toxic therapies that often include stem cell transplantation. Most AML patients are middle-aged or elderly and often are not candidates for aggressive therapies. There is therefore an acute need for new agents to treat AML.
Multiple Myeloma
SEER estimates indicate that 32,270 cases of multiple myeloma (MM) were diagnosed in the United States in 2020, with an estimated 12,830 deaths. While major advances in the treatment of the disease have occurred over the past decade, it is still considered an incurable disease. Treatments are often associated with significant toxicity and frequently involve a stem cell transplants. New agents are therefore clearly needed for the treatment of this disease.